Target volume definition
Intensity-modulated radiotherapy and image-guided radiotherapy allow us to deliver radiation precisely to the tumor and conform the dose distribution to complex shaped target volumes. Nowadays, the largest uncertainty is often the definition of the target volume, i.e. the region that should be irradiated. Radiotherapy planning distinguishes three target volumes:
- The gross tumor volume (GTV) comprising the macroscopic tumor mass
- The clinical target volume (CTV) containing microscopic extensions of the tumor
- The planning target volume (PTV) compensating for setup uncertainty and motion
GTV definition improved with the development of novel biomedical imaging techniques such as novel PET tracers and functional MRI. PTV margins were reduced through modern image guidance. Arguably, the definition of the CTV has made the least progress over the years. GTV definition amounts to delineating an abnormal tumor mass that es visible on imaging. In contrast, the CTV is invisible as it consists of microscopic tumor infiltration into normal appearing functioning tissues. Therefore, CTV definition is conceptually different from GTV definiton and is based on knowledge of the anatomically defined routes of tumor progression.
CTV definition has long been considered the domain of radiation oncologists and has attracted relatively little attention in the medical physics community. In our research group we work on computational methods to support target volume definition. Current research focuses on lymphatic cancer progression; past research projects considered brain tumors. This publication summarizes the role of computational methods for improving CTV definition:
- J. Unkelbach, T. Bortfeld, CE. Cardenas, V. Gregoire, W. Hager, B. Heijmen, R. Jeraj, S. Korreman, R. Ludwig, B. Pouymayou, N. Shusharina, J. Söderberg, I. Toma-Dasu, EGC. Troost, E. Vasquez Osorio. The role of computational methods for automating and improving clinical target volume definition. Radiotherapy and Oncology, 153:15-25, 2020
This presentation given remotely at the annual ESTRO meeting 2020 provides an overview on how computational methods may improve or automate the definition of the clinical target volume (CTV).
Elective nodal CTV definition for head & neck cancer
Head & neck squamous cell carcinoma (HNSCC) spread through the lymphatic system and form metastases in regional lymph nodes. Target volume definition involves detecting macroscopic metastases based on CT, PET, and MR imaging. However, in current practice a much larger part of the lymph drainage region is irradiated, which may harbor microscopic metastases despite negative imaging findings. In one of our main research projects, we work on a better quantification of the risk that parts of the lymph drainage system harbor occult metastases. This may lead to smaller and more personalized nodal CTVs.
The work includes 3 main areas:
- We collect large multi-institutional datasets on lymph node metastases in HNSCC patients to quantify the patterns of lymphatic metastatic progression. The web-based platform LyProX.org was developed to publicly host, share, and visualize the data.
- Based on these data, we develop statistical models for the lymphatic progression of tumors using the methodology of Hidden Markov Models (HMM), which predict the risk of occult metastases given the clinical diagnosis of a patient.
- Our work is translated into a phase II clinical trial on Personalized volume-deescalated elective nodal irradiation for oropharyngeal squamous cell carcinoma (DeEscO) where we irradiated much smaller volumes compared to current guidelines.
This video provides a brief introduction to radiotherapy target volume defintion for HNSCC:
Introduction to AI for target volume definition in head & neck cancer
1. Data curation and LyProx
Large datasets on lymph node involvement are the basis for a better quantification of the risk of occult metastases. We have collected approximately 3000 patients from 7 institutions in whom lymph node involvement was reported in detail. For all patients, involvement of each neck lymph node level is reported together with patient and tumor characteristics including T-stage, subsite, and lateralization. The web-based platform LyProX.org was developed to make the data publicly available. The graphical user interface allows users to filter that dataset for patient characteristics of interest (Figure 1).
Figure 1: GUI of LyProX for the exploration of datasets. In the example, the user has selected lateralized HNSCC located in the oropharynx that do not cross the midsagittal plane with involvement of ipsilateral level II. The GUI shows the percentage of patients with involvement of the other lymph node levels. Further instructions can be found on the website LyProX.org.
- R. Ludwig, J. Hoffmann, B. Pouymayou, M. Broglie Däppen, G. Morand, M. Guckenberger, V. Grégoire, P. Balermpas; J. Unkelbach. Detailed patient-individual reporting of lymph node involvement in oropharyngeal squamous cell carcinoma with an online interface. Radiotherapy & Oncology, 169:p1-7, 2022
2. Statistical models of lymphatic progression
Based on the data, we develop statistical models of lymphatic tumor progression to estimate the probability of occult metastases in clinically negative lymph node levels (LNL). To that end, we apply traditional statistical machine learning techniques, namely Hidden Markov models. Each LNL is represented as a binary node in a graphical model, which indicates if the LNL harbors metastases or not. Lymphatic metastatic spread is represented by arcs connecting the nodes. Arcs are associated with probabilities for the tumor to spread to or between LNLs, which can be learned from the datasets.
Figure 2: Representation of lymphatic metastatic progression via a graphical model.
This video provides a brief introduction to the statistical model:
Modeling lymphatic progression of cancer
Prior publications:
- Ludwig R, Pérez Haas Y, Benavente S, Balermpas P, Unkelbach J. A probabilistic model of bilateral lymphatic spread in head and neck cancer. Sci Rep. 2025 May;15(1):18152
- Ludwig R, Schubert AD, Barbatei D, Bauwens L, Hoffmann JM, Werlen S, Elicin O, Dettmer M, Zrounba P, Pouymayou B, Balermpas P, Grégoire V, Giger R, Unkelbach J. Modelling the lymphatic metastatic progression pathways of OPSCC from multi-institutional datasets. Sci Rep. 14(1):15750, 2024
- Pérez Haas Y, Ludwig R, Looman EL, Grégoire V, Balermpas P, Unkelbach J. Modelling occult lymph node metastases in HNSCC patients with a trinary state hidden Markov model. Phys Med Biol. 2025 Apr;70(8). doi: 10.1088/1361-6560/adc235
- R. Ludwig, B. Pouymayou, P. Balermpas, J. Unkelbach. A hidden Markov model for lymphatic tumor progression in the head & neck. Scientific Reports, 11(1):p1-17, 2021
- Pouymayou B, Balermpas P, Riesterer O, Guckenberger M, Unkelbach J. A Bayesian network model of lymphatic tumor progression for personalized elective CTV definition in head and neck cancer. Phys Med Biol. 2019;64(16):165003
3. Clinical trial on personalzed CTV-N definition
Based on this model, we have started the clinical trial "Personalized volume-deescalated elective nodal irradiation for oropharyngeal squamous cell carcinoma (DeEscO)". The trial is registered on ClinicalTrials.gov (NCT06563362). In total 6 Swiss institutions participate and a total of 120 patient will be enrolled. The trial is designed such that the estimated cumulative probability of occult metastases in all unirradiated LNLs combined is below 10%. This leads to substantial CTV-N reductions compared to the current guidelines as illustrated in Figure 3. Dose prescription and treatment of gross disease is according to standard-of-care.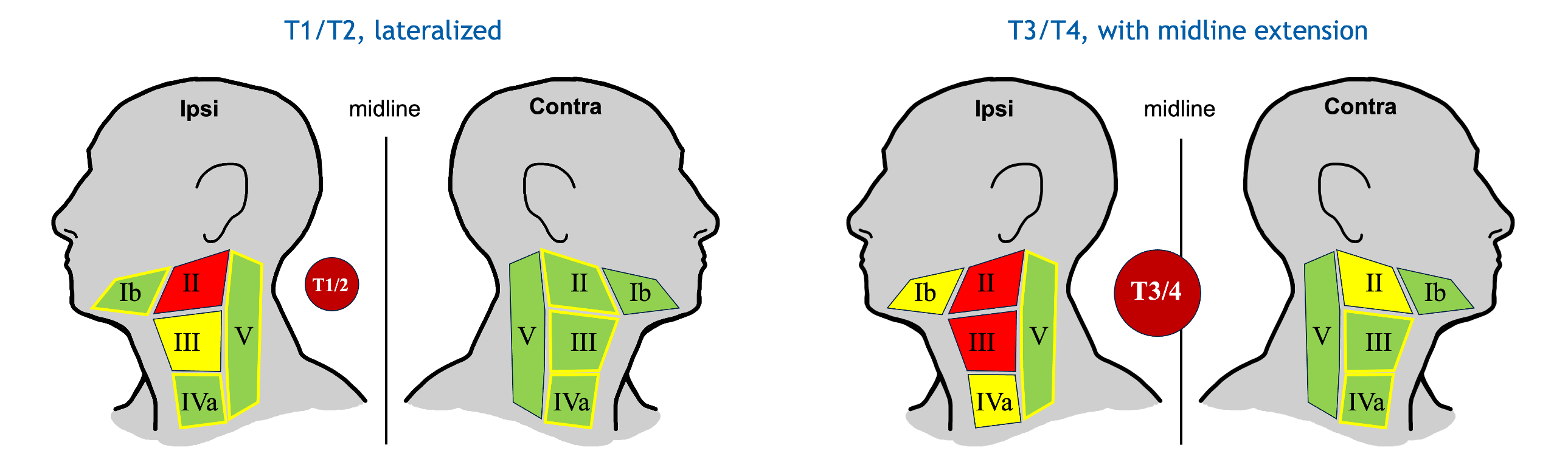
Figure 3. CTV-N definition in the DeEscO trial for two typical patients. Clinically involved LNLs are colored in red, the elective CTV-N is shown in yellow, and green colored levels are not irradiated. (left): For a lateralized early T-category tumor and clinical involvement of LNL II, only LNL III is electively irradiated. (right): For an advanced T-category tumor extending over the midline with involvement of LNLs II and III, levels Ib and IVa are irradiated. Contralateral irradiation is limited to LNL II. According to current guidelines, both patients would be treated the same: ipsilateral LNLs Ib-V and contralateral levels II-IV would be irradiated (as indicated by the yellow outlines).
- E. L. Looman et al., “Personalized volume-deescalated elective nodal irradiation in oropharyngeal squamous cell carcinoma (DeEscO): a study protocol,” Jun. 24, 2025, medRxiv. doi: 10.1101/2025.06.24.25330129
CTV definition for glioblastoma
Glioblastoma are known to infiltrate the healthy appearing brain tissue far beyond the tumor mass that is visible on MR imaging. However, the spatial patterns of tumor infiltartion are determined by the complex anatomy of the brain. Tumor cells spread primarily along white matter fibers while the falx and the ventricles represent anatomical barriers. In a previous project, we worked on phenomenological tumor growth models to facilitate automatic CTV definition that is consistent with neuroanatomy.
- J. Unkelbach, B. H. Menze, E. Konukoglu, F. Dittmann, M. Le, N. Ayache, and H. Shih. Radiotherapy planning for glioblastoma based on a tumor growth model: improving target volume delineation. Phys. Med. Biol., 2014; 59(3):747-770
- J. Unkelbach, B. H. Menze, E. Konukoglu, F. Dittmann, N. Ayache, and H. Shih. Radiotherapy planning for glioblastoma based on a tumor growth model: implications for spatial dose redistribution. Phys. Med. Biol., 2014; 59(3):771-790

