Combined proton-photon radiotherapy
Proton beams are widely considered a superior radiation modality compared to high energy xrays for treating cancer patients. This is due to the favorable depth dose curve of protons, which deposit most of the energy at the Bragg peak. Instead, photons show an exponential falloff of dose as a function of depth inside the patient.
However, proton therapy is a limited resource due to cost and size of the facility. Currently, there are only in the order of 100 proton therapy centers worldwide compared to more than 10'000 conventional xray therapy units. In our research group we work on two aspects in that regard:
- We develop novel cost-effective proton therapy concepts that may allow for a more widespread implementation of proton therapy.
- We develop methods to optimally make use of limited proton therapy resources, both for individual patients but also for the population of all cancer patients.
Our approach to these problems is combined proton-photon radiotherapy, that is, we work on methods to optimally combine proton beams with conventional x-ray beams.
For an overview of our work on combined proton-photon radiotherapy, you may watch this presentation given at the AAPM annual meeting 2021.
Combined proton-photon radiotherapy using a fixed proton beamline
The size and cost of the proton gantry is one of the main obstacles for a widespread implementation of proton therapy. Due to the size of the gantry, proton therapy can usually not be installed in existing radiotherapy departments designed for xray therapy units. However, installing a cyclotron and a fixed proton beamline may be possible in many situations. In our work, we explore the following concept: The treatment room consists of:
- A Linac for delivering intensity-modulated x-ray therapy (IMRT, VMAT)
- A robotic couch for treatment in lying position
- A fixed proton beam line equipped with pencil beam scanning
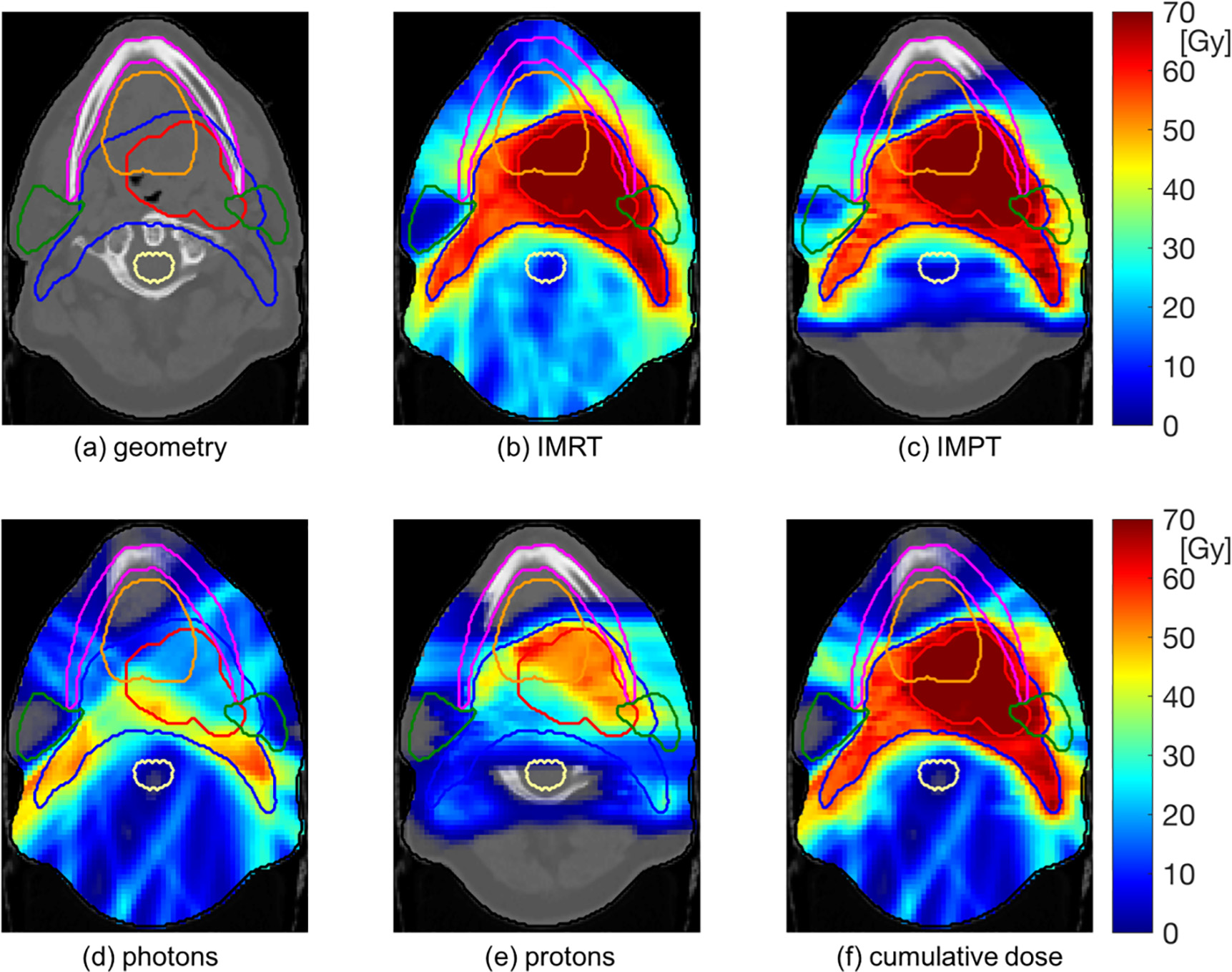
With this concept, protons and photons may be delivered in the same fraction with the same patient immobilization. Most of the dose may be delivered with protons to reduce the dose burden in normal tissues. However, were protons alone are suboptimal due to limitations in the available beam angles, this can be compensated for by photon beams to achieve optimal conformity. The concept is illustrated for a head & neck cancer patient in Figure 1.
Figure 1: Illustration of a combined proton-photon treatment (d-f) for a head & neck cancer patient. Most of the dose to the gross tumor volume (red contour) is delivered by protons. However, horizontal proton beams are suboptimal for minimizing dose to the parotid glands (green contours). Photon beams therefore improve conformity of the dose distribution around the parotid glands and the nodal target volume (blue contour).
Further details can be found in our recent publications.
- Fabiano S, Balermpas P, Guckenberger M, Unkelbach J. Combined proton–photon treatments - A new approach to proton therapy without a gantry, Radiotherapy and Oncology 145, 81-87, 2020
- L. Marc, S. Fabiano, N. Wahl, C. Linsenmeier, A. Lomax, J. Unkelbach. Combined proton-photon treatment for breast cancer. Phys. Med. Biol., 66(23):235002, 2021
- F. Amstutz, S. Fabiano, L. Marc, D. Weber, A. Lomax, J. Unkelbach, Y. Zhang. Combined proton‐photon therapy for non‐small cell lung cancer, Medical Physics, 2022
Optimally combining proton and photon fractions
Currently, protons and photons are not available in the same treatment room. For the time being, the most practical way to combine protons and photons is to deliver some fractions with protons and the rest with photons. In this case, two questions arise:
- How can a limited number of proton therapy fractions be optimally allocated in a population of cancer patients to maximize the benefit of limited proton therapy resources for the healthy care system as a whole? How many proton fractions should each patient receive?
- If only a subset of fractions is delivered with protons, how can a limited number of proton fractions be best exploited for an individual patient to optimize treatment quality?
In previous research projects, we have worked on these questions.
Optimal proton slot allocation
We have considered a cohort of Head & Neck cancer patients. The Netherlands and Denmark have implemented schemes to select patients for proton therapy based on the expected benefit regarding normal tissue complication probability (NTCP). We have asked the question if the average NTCP for a population of Head & Neck cancer patients can be reduced by combining proton and photon fractions. Thereby, limited proton slots are distributed over a larger set of patients compared to a patient selection scheme where patients receive either protons or photons.
- N. Loizeau, S. Fabiano, D. Papp, K. Stützer, A. Jakobi, A. Bandurska-Luque, E. Troost, C. Richter, J. Unkelbach. Optimal allocation of proton therapy slots in combined proton-photon radiotherapy. Int. J. Rad. Onc. Biol. Phys., 111(1):p196-207 , 2021
The results show that, in principle combined proton-photon treatments can improve on patient selection schemes. However, the NTCP reduction for the population is small if protons and photons are required to deliver the same dose per fraction to the tumor.
In a follow-up publication, we considered stereotactic body radiotherapy of liver cancer patients. In this case, proton and photon fractions do not necessarily have to deliver the same dose per fraction. A small number of proton fractions can be overproportionally exploited if protons deliver a larger dose per fraction than photons. Thereby, combined proton-photon treatments may provide a substantial advantage over patient selection schemes.
- Marc L, Unkelbach J. Optimal use of limited proton resources for liver cancer patients in combined proton-photon treatments. Phys Med Biol. 2025 Jan;70(2). doi: 10.1088/1361-6560/ad94c8
Joint optimization of proton and photon fractions
Institutions that currently perform multi-modality treatments optimize intensity modulated radiation therapy (IMRT) and proton therapy (IMPT) plans separately so that each modality delivers the prescribed dose per fraction to the target volume. Our group has demonstrated that one can improve on such simple combinations by simultaneously optimizing IMRT and IMPT plans.
The optimal combination of proton and photon fractions has to make a trade-off between two conflicting goals. To best exploit the lower normal tissue dose of protons, one should deliver an overproportionate dose with protons, i.e. increase the dose per fraction compared to photons. However, if all the dose was delivered in a few proton fractions, the effect of fractionation is reduced. To protect normal tissues surrounding the tumor through fractionation, some dose should be delivered in the photon fractions. The optimal tradeoff can be found by simultaneously optimizing proton and photon fractions based on their cumulative biologically effective dose (BED).
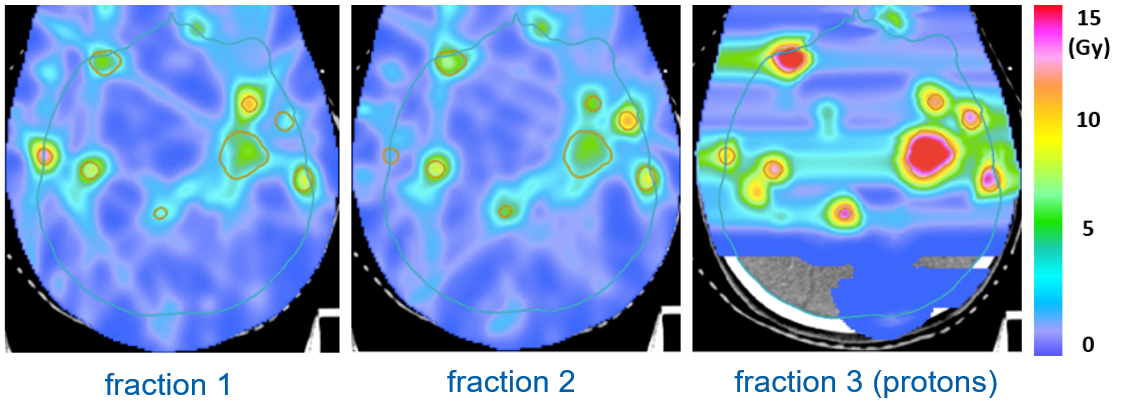
Figure 2 illustrates this for a patient with multiple brain metastases treated in 3 fractions. Two fractions are delivered with photons and one fraction is delivered with protons. The goal is to minimize the BED2 in the normal brain for a given prescribed cumulative BED10 delivered to the metastases. In the optimized combination of proton fractions, approximately half the dose is delivered in the single proton fraction. The optimal proton and photon doses are determined automatically through the treatment plan optimization algorithm and vary between the metastases.
Figure 2 : Combination of 2 photon fractions and 1 proton fraction for a patient with multiple brain metastases.
Further details can be found in our publications:
- Torelli N, Bicker Y, Marc L, Fabiano S, Unkelbach J. A new approach to combined proton-photon therapy for metastatic cancer patients. Phys Med Biol. 69(14), 2024
- Unkelbach J, Bangert M, De Amorim Bernstein K, Andratschke N, Guckenberger M. Optimization of combined proton-photon treatments. Radiother. Oncol. 128(1):133-138, 2018
- Fabiano S, Bangert M, Guckenberger M, Unkelbach J. Accounting for range uncertainties in the optimization of combined proton-photon treatments via stochastic optimization. Int. J. Rad. Onc. Biol. Phys. 108(3), 792-801, 2020